what ester is formed from ethanol and ethanoic acid Esters in food
Alcohols, Carboxylic Acids and Esters - Understanding their Structures When it comes to the world of chemistry, there are certain substances that are commonly used in daily life, and yet, not everyone takes the time to understand their structures. These substances include alcohols, carboxylic acids, and esters. Alcohols, as we know, are commonly used in the form of beverages. But what exactly are alcohols? They are organic compounds that contain a hydroxyl (-OH) group, which is bonded to a carbon atom. This hydroxyl group gives alcohols their characteristic properties, such as being able to evaporate quickly and having a distinctive odor. Carboxylic acids, on the other hand, are organic compounds that contain a carboxyl group (-COOH), which is also bonded to a carbon atom. These compounds can be found in various forms - from acids that are commonly used in cooking, such as acetic acid (vinegar), to carboxylic acids that are used to produce perfumes and other fragrances. Lastly, we have esters. Esters are organic compounds that are commonly used in the production of fragrances and flavorings. They contain an oxygen atom that is bonded to a carbon atom, which is in turn bonded to another oxygen atom. Esters are formed from the reaction between alcohols and carboxylic acids, and they are known for their sweet and fruity aromas. Now that we have a basic understanding of these substances, let’s take a closer look at their structures through the help of some excellent resources. The first resource, “Print Chemistry (Chapter 11)- Structures of alcohols, carboxylic acids”, provides an in-depth look at the molecular structures of both alcohols and carboxylic acids. The images in this resource are particularly useful, as they help to illustrate the bonding patterns that exist between atoms in these substances. For example, the image for ethanol (a type of alcohol) shows that there are two carbon atoms (C) bonded to each other, and each of these carbon atoms is also bonded to three hydrogen atoms (H). Additionally, one of the carbon atoms is bonded to an oxygen atom (O), which is where the hydroxyl group (-OH) is located. The second resource, “BBC - GCSE Bitesize Science - Esters : Revision”, focuses specifically on esters. This resource provides an excellent overview of the process by which esters are formed, as well as their unique structures. The image for ethyl ethanoate (a type of ester) shows that it is formed from the reaction between ethanol (an alcohol) and ethanoic acid (a carboxylic acid). Additionally, the image shows that the ester has a distinct structure, with an oxygen atom (O) bonded to a carbon atom (C), which is in turn bonded to another oxygen atom (O). In conclusion, understanding the structures of alcohols, carboxylic acids, and esters can provide insight into their properties and uses. Resources such as the “Print Chemistry” and “BBC - GCSE Bitesize Science” guides are excellent tools to help visualize the structures of these substances. By taking the time to learn about these compounds, we can truly appreciate the role they play in our everyday lives.
If you are searching about Esters in Food - Chemistry LibreTexts you’ve came to the right place. We have 5 Images about Esters in Food - Chemistry LibreTexts like BBC - GCSE Bitesize Science - Esters : Revision, Esters in Food - Chemistry LibreTexts and also Print Chemistry (Chapter 11)- Structures of alcohols, carboxylic acids. Here you go:
Esters In Food - Chemistry LibreTexts
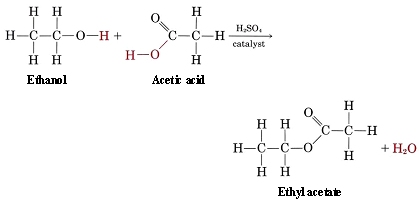 chem.libretexts.orgesters ester libretexts ethyl equation condensation carboxylic
chem.libretexts.orgesters ester libretexts ethyl equation condensation carboxylic
Print Chemistry (Chapter 11)- Structures Of Alcohols, Carboxylic Acids
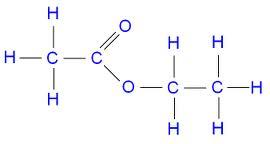 www.easynotecards.comethanoate ethyl structure draw science acid ester ethanoic methyl propyl butyl esters class compound gcse print question cbse sa2 paper
www.easynotecards.comethanoate ethyl structure draw science acid ester ethanoic methyl propyl butyl esters class compound gcse print question cbse sa2 paper
Carbon And Its Compounds
 transitiononweb.blogspot.comacid reaction ester form base ethanoic class reacts its 10th acetic strong used smell
transitiononweb.blogspot.comacid reaction ester form base ethanoic class reacts its 10th acetic strong used smell
An Introduction To Esters
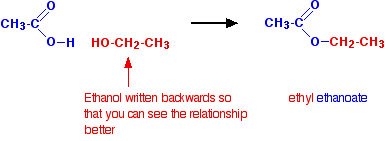 www.chemguide.ukesters acid ethanol ester ethanoic equation water properties between diagram chemguide organic chemistry intended isn libretexts fats gif organicprops
www.chemguide.ukesters acid ethanol ester ethanoic equation water properties between diagram chemguide organic chemistry intended isn libretexts fats gif organicprops
BBC - GCSE Bitesize Science - Esters : Revision
 www.bbc.co.ukethanoate ethyl acid ethanoic ester esters sulfuric chemistry ethanol reaction bbc water gcse formation reacts sodium diagram organic presence chemical
www.bbc.co.ukethanoate ethyl acid ethanoic ester esters sulfuric chemistry ethanol reaction bbc water gcse formation reacts sodium diagram organic presence chemical
Esters in food. An introduction to esters. Ethanoate ethyl acid ethanoic ester esters sulfuric chemistry ethanol reaction bbc water gcse formation reacts sodium diagram organic presence chemical